The estimated glucose threshold (eTmG/GFR) - a helpful tool in SGLT2 Inhibition?
Assessing tubular function by measuring urinary excretions of different substances is an important albeit somewhat dissipating part of clinical nephrology.
Gradient-limited versus transport-limited tubular reabsorption
Fig. 1. Formulas for calculating the fractional excretion (FE) and the fractional tubular reabsorption (TR) of a substance Z.
Absolute excretion rates and indices like the fractional excretion (FE) or its counterpart the tubular reabsorption (TR) are commonly used to assess tubular function (Fig. 1).
These are quite informative for substances that are mainly “gradient-limited” (e.g. sodium), where the reabsorptive capacity is confined by the maximal concentration gradients, that tubular cell membrane channels and tight junctions can sustain. Here absolute and relative excretion rates are mainly dependent on and as such representative of tubular function itself.
Fig. 2. The filtered load (FL) of a sustance Z, the amount filtered by the glomeruli, is given by the above formula.
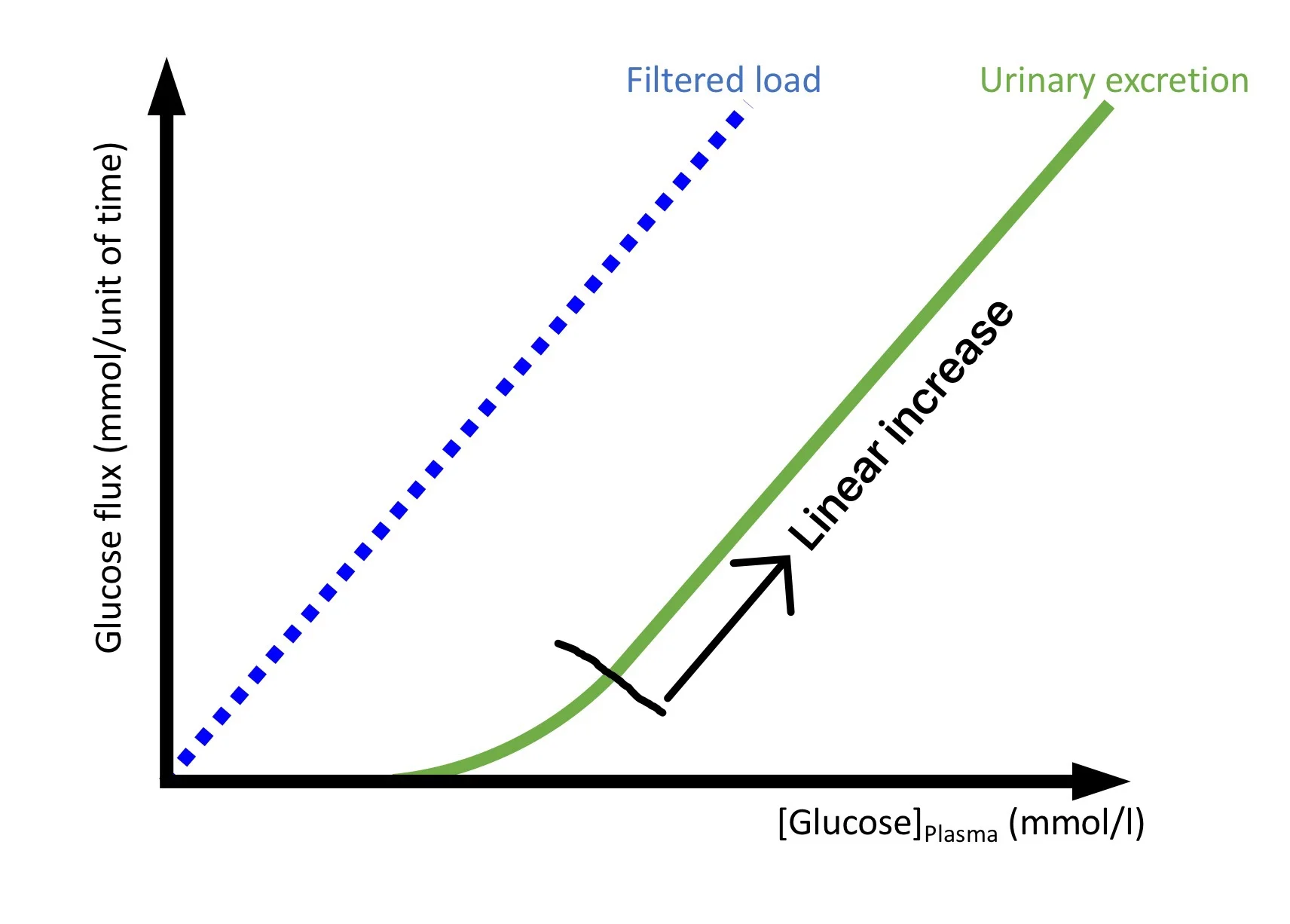
This is different with “transport-limited” tubular reabsorption (e.g. glucose, phosphate). These processes are enzyme-based, often follow Michaelis-Menten kinetics and exhibit an absolute upper limit to the amount that can be reclaimed from the tubular lumen. Therefore the urinary excretion of these substances is not only dependent on tubular function, but also strongly influenced by the filtered load (FL), i.e. by the GFR and the respective plasma concentration of the particular solute (Fig 2).
Fig 3.
The theoretical relationship between filtered load and urinary excretion of transport-limited substances is shown in Fig. 3.
The glucose threshold
“In normal individuals as the concentration of glucose in the plasma rises the amount [of glucose] excreted [in the urine] remains extremely small till a limiting plasma concentration, the threshold, is reached. As the concentration in the blood rises above the threshold, progressively more glucose is excreted” (1).
The threshold concentration of glucose (and any other substance) can be reckoned by dividing the tubular transport maximum of glucose by the GFR (Fig 4). It represents the fraction of the plasma glucose concentration whose filtration can be maximally reabsorbed by the tubule. It has therefore also been called the aglucosuric glucose concentration (2).
Fig. 4. How to derive the glucose threshold concentration (TmG/GFR) from the filtered load (FL). The rectangular brackets [ ] signify concentrations.
By accepting the creatinine clearance as a surrogate of the GFR it can be easily estimated by measuring the concentrations of glucose and creatinine in a plasma and a spot urine sample respectively (Fig. 5).
Using this estimated tubular threshold concentration is a great way to characterize tubular handling of glucose (and other transport-limited substances) because it eliminates the filtered load from the equation and expresses only tubular function itself.
Fig. 5. Estimating the tubular threshold for glucose (eTmG/GFR). FE fractional excretion, TR tubular reabsorption.
How to deal with splay
Due to heterogeneity in the number and affinity of glucose transport sites, the maximal tubular transport capacity of singular nephrons extends over a certain range. This means that some nephrons become glucosuric at lower concentrations than others. Only after the transport maximum of all individual nephrons is exceeded does the urinary excretion get proportional to the filtered load (Fig. 6a). The usually small amounts excreted before that have been called splay (Fig. 6b).
Fig. 6a.
Fig. 6b.
Fig. 6c.
“Extrapolation of [the linear part of the urinary excretion line] to its point of intersection with the abcissa yields [the so called ‘line’ threshold (Fig. 6c)], … [the] value of blood glucose at which excretion would begin were there no splay” (2).
The estimated glucose threshold represents a good approximation of this theoretical (‘line’) threshold, as long as the splay comprises only a small part of the whole quantity of glucose excreted. Predicting wether this is the case in an individual patient might not be trivial. I am not aware of any empirically validated approaches and adaptations to specific clinical situations might be needed. I would suggest the following aids:
In analogy to work done with phosphate (3), I would consider it safe to ignore splay when the fractional excretion of glucose is above 15%.*
The higher the absolute amount of glucose excreted, the more dependable the estimated threshold becomes.
In cases where splay is quantitatively significant the estimated threshold always underestimates the theoretical one.
Using the threshold in SGLT2 inhibition
SGLT2 inhibitors interfere with glucose reabsorption in the proximal tubule. Their clinical effectiveness probably correlates with the absolute amount of glucose excreted. As explained above their clinical impact is therefore not only dependent on SGLT2 inhibition itself, but also strongly influenced by the glycemic control of the individual patient. The estimated glucose threshold, which eliminates the impact of glycemia, could thus be an important tool to better analyze the pharmacodynamic effects of the SGLT2 inhibitors.
I would expect the eTmG/GFR to be a helpful tool
to improve adherence monitoring
to allow dose titration in the individual patient
to improve safety (eg by reducing the risk if ketogenesis)
Outlook
Clearly, estimating the tubular threshold of glucose is not rocket science and empirical validation of the concept is needed. Nonetheless I think it has the potential to be clinically helpful and I would love to see it evaluated in clinical practice.
References
Woolf LI, Goodwin BL, Phelps CE. Tm-limited renal tubular reabsorption and the genetics of renal glucosuria. Journal of Theoretical Biology. 1966;11(1):10–21.
Smith HW. The kidney: Structure and function in health and disease. New York: Oxford University Press; Dezember 1, 1951: 81-96.
Payne RB. Renal tubular reabsorption of phosphate /TmP/GFR): indications and interpretation. Ann Clin Biochem. 1998;35:201-206.
DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrology. 2017; 13:11-26.
* This should work in patients treated with dapaglifozin and empaglifozin where splay is reduced (4), also in glucosuric diabetic and nondiabetic patients not treated with SGLT2 inhibitors, and patients with familial renal glucosuria type A. More caution is probably needed in patients with familial renal glucosuria type B and possibly in patients treated with other SGLT2 inhibitors where splay might be increased. Nonetheless with high enough glucose excretion the estimation should work in these cases as well.



![Fig. 4. How to derive the glucose threshold concentration (TmG/GFR) from the filtered load (FL). The rectangular brackets [ ] signify concentrations.](https://images.squarespace-cdn.com/content/v1/546d1156e4b03a986e5d6015/1492499208577-RMRBSRQK3717F63KOKKU/Derivation+TmG%2FGFR+%40swissnephro+Florian+Buchkremer)